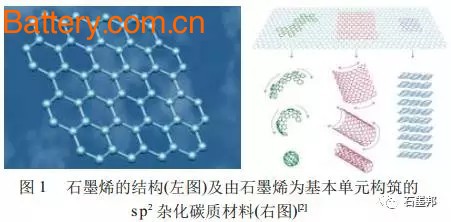
Energy and environmental issues are two major issues that humans need to solve today. Today, with the depletion of fossil energy, increasing environmental pollution, and global warming, it is particularly urgent to seek renewable green energy instead of traditional fossil energy and to seek harmony between people and the environment. The use of new types of renewable energy sources, such as wind and solar energy, the gradual marketization of electric vehicles and hybrid electric vehicles , and the rapid development of various portable electrical devices require efficient, practical and “green†(zero pollution, low). Energy storage and transportation system. For the new “green†energy storage devices, while considering their “greenâ€, high power density and high energy density are important indicators for whether they can truly replace the traditional energy storage and transportation system. New power systems, especially secondary batteries or supercapacitors, are currently important "green" energy storage devices. The core part is the energy storage material with excellent performance. Various carbonaceous materials, especially sp2 hybrid carbonaceous materials, become important energy storage materials or electrode materials for energy storage systems due to their special layered structure or large specific surface area. Graphene, a single-layer graphite structure of sp2 hybrid carbonaceous materials, was successfully prepared in 2004; unique structure - true surface solids (non-porous, surface carbon atom ratio is 100%) The oversized surface material makes it an important choice for the next generation of carbonaceous electrode materials. 1 sp2 hybrid carbonaceous material: an important energy storage material Carbon is an element widely found in nature and is characterized by diversity, specificity and extensiveness. The carbon element can form a solid element by three hybrid methods: sp, sp2 and sp3. The basic structure of the carbonaceous material formed by sp2 hybridization is a two-dimensional graphene sheet. As shown in Figure 1, if there is a five-membered ring lattice in the graphene lattice structure formed by the six-membered ring, the graphene sheet will be warped, and when there are more than 12 five-membered ring lattices Zero-dimensional fullerene is formed; carbon nanotubes can be regarded as cylindrical one-dimensional materials formed by graphene curling at a certain angle; graphene sheets interact and superimpose to form three-dimensional bulk graphite. As amorphous porous carbonaceous materials (activated carbon, activated carbon fiber, carbon aerogel, etc.), it is formed by the interaction of defect-rich microcrystalline graphite carbon (thickness and small-scale three-dimensional graphite sheet structure). Carbonaceous materials are currently one of the most widely used electrode materials in green power systems. In the new energy fields such as lithium ion secondary batteries, supercapacitors , solar cells, fuel cells, hydrogen storage/methane, there are everywhere carbon materials. The sp2 hybrid carbonaceous material has a layered structure of graphite (or a small-scale microcrystalline graphite) or a texture feature (rich pores) formed by a large number of defects and a large specific surface area, and becomes an important electrode material. The materials mainly include: graphite materials, porous carbon materials and carbon nanotubes. The layered sp2 carbon graphite material with less defects is the most widely used commercial lithium ion battery anode material; the porous carbon material rich in defects is the main electrode material of the current supercapacitor; and the carbon nanotube as a novel sp2 Hybrid carbonaceous materials are predicted to be widely used in dye-sensitized solar cells. Regardless of the commercialization or "green" energy storage devices still in the research and development stage, its performance and cost performance need to be improved, structural optimization and modification of sp2 hybrid carbonaceous materials, development of higher performance or more cost-effective electrode materials. It is the mission of materials scientists. Taking supercapacitors as an example, the development of higher power density, higher energy density, and cost-effective carbonaceous electrode materials is a task that materials scientists must accomplish before they are actually going to large-scale applications. The author believes that in the research and development of carbon-based supercapacitor materials, materials scientists can work from the following aspects: (1) Expansion of storage space - high energy density The storage mechanism of a carbon-based electric double layer capacitor is an ordered enrichment of charge on the surface of the electrode. For supercapacitors, the larger the "surface area" suitable for charge accumulation (the surface to which the electrolyte solution can contact), the greater the storage capacity. The limiting specific surface area (single-layer graphene sheet) of the defect-free sp2 carbonaceous material is 2 630 m2/g; and the limit specific surface area of ​​the defect-rich sp2 carbonaceous material is larger than this value. Since the general method is difficult to obtain a single-layer graphene sheet, the main method for increasing the specific surface area of ​​the carbonaceous material is to create pores in the carbonaceous material, increase the proportion of surface carbon atoms, thereby increasing the specific surface area; and the increase of porosity restricts A further improvement in its power characteristics. How to improve the specific surface area and obtain high energy density while maintaining high power characteristics is an important issue in obtaining high performance supercapacitors. (2) Control of microstructure and macro texture - high power characteristics In general, a high specific surface area carbonaceous electrode material is obtained mainly by increasing the porosity. However, the existence of pores brings about another problem, that is, the problem of diffusion of the electrolyte solution. How to improve the specific surface area while maintaining the infiltration of the electrolyte solution on the electrostatic charge storage surface, and ensure that the electrolyte ions diffuse from the solution body to the surface of the carbonaceous material at a relatively high rate, which is one of the important problems to be solved in the carbonaceous electrode material. . (3) Improve the structural integrity of graphene sheets - low internal resistance and high electrical conductivity The electrode material requires good electrical conductivity and the complete graphene sheet has good electrical conductivity. The sp2 carbonaceous material as the electrode material should have good structural integrity. The pore-defect is created by activation or the like, and the conductivity characteristic is deteriorated while the specific surface of the carbonaceous material is increased. How to improve the specific surface area without reducing the conductive properties of sp2 carbon is also a bottleneck that needs to be overcome to improve the performance of carbonaceous electrode materials. As a single layer or thin layer of graphene with sp2 hybrid carbonaceous material, it is an ideal material to solve the above bottleneck. The main reasons are as follows: single or several layers of graphene sheets with a two-dimensional planar structure without voids. The storage space is located on the surface of the graphene sheet, and its energy storage characteristics are completely dependent on the specific surface area and surface chemistry of the graphene. The micron-sized graphene sheets overlap to form a graphene macroscopic body, which has simple texture characteristics, no pores, and good contact with the electrolyte solution. After being compounded with other materials, the texture can be adjusted to ensure good power properties of the material. If used as a negative electrode material for lithium ion batteries, the diffusion path of lithium ions between thin layers of graphene sheets (the sheet size is on the order of micrometers, much smaller than that of bulk graphite) is relatively short, and the power characteristics can be greatly improved. The graphene sheet has zero defects or few defects, ensuring good electrical and thermal conductivity properties, and is an ideal candidate for electrode materials, especially for electrode materials used in miniature power devices. Based on the above points, a single layer or a thin layer (2 to 10 layers) of graphene as an sp2 hybrid material is an ideal supercapacitor electrode material, and it is expected to improve the power and energy density of the supercapacitor. At the same time, graphene is also an ideal candidate for other energy storage systems due to its unique thin layer, longitudinal and lateral scale cuttability, good thermal and electrical properties. 2 sp2 carbonaceous material's elementary material - graphene: birth and odd properties In 2004, the Geim team at the University of Manchester first obtained monolayer and thin-layer graphene using mechanical cleavage. Prior to this, scientists have always believed that strict two-dimensional crystals are thermodynamically unstable and cannot exist independently. Graphene is the thinnest two-dimensional material known at present. The perfect graphene has an ideal two-dimensional crystal structure and is composed of a hexagonal lattice. Since it was successfully prepared, graphene has caused a new research boom around the world. Physics, chemistry, and materials scientists have begun systematic research on graphene, and various fascinating and unique properties have been discovered. Forecasts are likely to cause revolutionary changes in many areas. At present, the main methods for preparing graphene include mechanical splitting, epitaxial crystal growth, chemical vapor deposition, thermal expansion and reduction of graphite oxide. Other preparation methods have also been developed, such as gas phase plasma growth technology, electrostatic deposition method and high temperature and high pressure synthesis method. The author believes that among these methods, it is most likely to realize the scale-up preparation of graphene, and the large-scale application is the thermal expansion method and reduction method of graphite oxide. The main process of this method is to rapidly heat the graphite oxide to a certain temperature or higher in a short time (the general method is above 1 000 ° C), so that the graphite oxide sheets are peeled off from each other by the decomposition of the functional groups between the sheets. The graphene oxide reduction method uses graphite oxide as a raw material and ultrasonically in a solvent to obtain a graphene oxide solution, which is then reduced with a chemical reducing agent to obtain graphene. A lot of existing research work is also based on these two methods. Our group invented the low-temperature thermal expansion technology to obtain macro-graphene materials at low cost. Graphene is a true surface solid. The ideal single-layer graphene has a large specific surface area (2 630 m2 / g) and is a potential energy storage material. Graphene also has good electrical, mechanical, optical and thermal properties. Graphene is a semiconductor with no energy gap. It has much higher carrier mobility than silicon (2×105 cm2 /V), and has a mean free path of micron order and a large coherence length at room temperature. Alkene is an ideal material for nano-circuits and an ideal material for verifying quantum effects. Graphene has good electrical conductivity, and its electrons move at a speed of 1/300 of the speed of light, far exceeding the speed of electrons in a general conductor. Graphene has good light transmittance and is a potential substitute for traditional ITO films. Graphene has good thermal properties, and Ghosh et al. measured the thermal conductivity of graphene from 3080 to 5150 W/mK using a non-contact technique based on micro-Raman spectroscopy. Graphene also has very high mechanical strength. Liu and Lee used the first principle calculation and experiment to prove that the graphene sheet is the most known material with the highest strength, and its ideal strength is 110~130GPa. Good electrical conductivity is a unique property that other large specific surface area carbonaceous materials are difficult to foresee, indicating that graphene is likely to be an excellent electrode material; good thermal conductivity, optical properties and mechanical strength also indicate graphite. The olefinic materials are useful in ultra-thin, ultra-micro electrode materials and energy storage devices, and such energy storage components can be used in high density nanoelectronic devices and high power battery packs. 3 Graphene with ideal two-dimensional structure: new energy storage materials 3.1 Application of graphene in supercapacitors Carbonaceous materials are the earliest and widely researched and widely used supercapacitor electrode materials. Carbonaceous materials for supercapacitors are currently mainly concentrated on activated carbon (AC), activated carbon fibers (ACF), carbon aerogels, carbon nanotubes (CNTs), and template carbon. The elementary material of these sp2 carbonaceous materials is graphene. Since the successful preparation of graphene, people began to explore the possibility of using this extreme structure of sp2 carbonaceous materials in supercapacitors. The Ruoff team tested the performance of graphene-based supercapacitors using chemically modified graphene as the electrode material. The capacitive properties of this graphene material can reach 135 F/g and 99 F/g in water and organic electrolytes, respectively (Figure 2). Rao et al. compared the capacitive properties of graphene prepared by three methods. In the sulfuric acid electrolyte, the graphene obtained by the graphite oxide thermal expansion method and the nano-diamond conversion method has a high specific capacitance, which can reach 117 F/g; in the organic electrolyte, when the voltage is 3.5 V, the specific capacitance The specific energy can reach 71F/g and 31.9 Wh/kg. Our group of graphene materials prepared by low-temperature thermal expansion method can achieve 180-230 F/g in a 30% (mass fraction) KOH electrolyte without any post-treatment; after compounding with oxide, specific capacitance It has been greatly improved while having good power characteristics. The Institute of Metals of the Chinese Academy of Sciences and the relevant group of Nankai University have also made good research progress. The use of graphene materials in supercapacitors has its unique advantages. Graphene is a completely discrete single-layer graphite material, and the entire surface thereof can form an electric double layer; however, in the process of forming macroscopic aggregates, the graphene sheets are superimposed on each other, which reduces the area of ​​forming an effective electric double layer ( The graphene obtained by the general chemical method has 200 to 1 200 m 2 /g). Even so, graphene can still obtain a specific capacitance of 100~230 F/g. If the surface can be completely released, a specific capacitance much higher than that of the porous carbon will be obtained. In the process of stacking graphene sheets and forming macroscopic bodies, the pores formed are concentrated above 100 nm, which is beneficial to the diffusion of electrolyte. Therefore, graphene-based supercapacitors have good power characteristics. 3.2 Application of graphene in lithium ion battery Research on anode materials for lithium ion batteries has focused on carbonaceous materials, alloy materials and composite materials. Carbonaceous materials are the first materials that have been researched and applied to the commercialization of lithium-ion batteries. They are still one of the focuses of attention and research. Carbonaceous materials can be classified into graphitizable carbon (soft carbon), amorphous carbon (hard carbon) and graphite according to their structural characteristics. At present, the research on carbon negative electrode mainly uses various means to modify its surface, but the surface treatment of artificial graphite will further increase the manufacturing cost. Therefore, the focus of future research will still be on how to better utilize cheap natural graphite. And develop valuable amorphous carbon materials. Therefore, it is the main research direction to manufacture low-cost and high-performance lithium ion battery anode materials from graphite. Graphene is a new type of carbonaceous material prepared from graphite. The application potential of single-layer or thin-layer graphite (2-10 layers of graphene) in lithium-ion batteries is also in the researcher's field of vision. Yoo et al. studied the performance of graphene in the anode material of lithium ion secondary batteries, and its specific capacity can reach 540 mAh/g. If C60 and carbon nanotubes are incorporated therein, the specific capacity of the negative electrode can reach 784 mAh/g and 730 mAh/g. Khantha et al. discussed the lithium storage mechanism of graphene through theoretical calculations. We use the graphene material prepared by the low temperature method directly for the anode material of the lithium ion secondary battery, and the first discharge specific capacity can reach 650 mAh/g. After modification, this result can be improved. However, its first charge and discharge efficiency and cycle efficiency are low, and it is necessary to modify the graphene structure. Since multilayer graphene has a certain lithium storage space and the diffusion path of lithium ions is relatively short, it should have better power characteristics. The relevant group is currently carrying out structural modification and compounding of graphene and conducting related research work. 3.3 Graphene in solar cells In addition to showing great potential as a supercapacitor and a lithium-ion battery , graphene also presents unique advantages in solar cells and gas storage. Two-dimensional graphene has good light transmittance and electrical conductivity and is a potential material to replace ITO. The use of graphene to make transparent conductive films and their application in solar cells has also become a hot spot of research. Wang et al. used graphene, which was reduced by thermal expansion of graphite oxide, to produce a transparent conductive film for use in dye-sensitized solar cells, and achieved good results. The prepared graphene transparent conductive film can have a conductivity of 550 S/cm, and the light transmittance can reach 70% or more in the wavelength range of 1000 to 3000 nm (Fig. 3). The graphene transparent conductive film prepared by Wu et al. by solution method is applied as an anode in an organic solar cell, but since the applied graphene is not effectively reduced, the electric resistance is large, resulting in short circuit current and filling factor of the obtained solar cell. Less than indium oxide, if the resistance of the graphene film can be lowered, the result may be better. The organic solar cells assembled by Liu et al. using a solution method prepared by a solution of graphene and other noble metal materials can have a short-circuit current of 4.0 mA/cm2, an open circuit voltage of 0.72 V, and a light conversion rate of 1.1%. Li et al. successfully prepared high-quality graphene by stripping-re-embedding-expansion method. The electrical resistance was 100 times lower than that of graphene prepared by using graphite oxide as raw material, and LB was successfully prepared with DMF as solvent. Membrane, this transparent conductive film has also become a potential material for solar cells. We and the cooperation team first reported the preparation of large surface area, unsupported ultra-thin graphene films by gas-liquid interface self-assembly method; after selective doping and modification, graphene flexible films with different electrical properties and transmittance can be obtained. It is a potential solar cell electrode material. 3.4 Application of graphene in hydrogen storage/methane Dimitrakakis designed a three-dimensional hydrogen storage model using graphene and carbon nanotubes. If this material is doped with lithium ions, it can store up to 41g/L at atmospheric pressure (Figure 4). Therefore, the emergence of new materials such as graphene provides a new idea and material for the design of hydrogen storage/methane materials. 4 Conclusion and Prospects——A Prospect Analysis of Graphene as a New Energy Storage Material Graphene has a large specific surface area, good electrical conductivity and thermal conductivity, and is a potential energy storage material. The author believes that graphene as an energy storage material has the following advantages: Graphite raw materials are abundant and cheap, and the cost of graphene prepared by chemical method is relatively low; the low-temperature expansion method invented by our research group has greatly reduced the cost. After optimizing and amplifying the process, the chemically prepared functional graphene material is expected to become a very competitive energy storage material. Graphene has good electrical conductivity and an open surface, giving it good energy storage power characteristics. The macroscopic texture is formed by overlapping micron-sized and conductive graphene sheets to form an open large-aperture system. Such a structure provides a very low barrier to the entry of electrolyte ions, ensuring the material. Good power characteristics. Graphene has a large theoretical specific surface area. The large specific surface area determines its high energy density. At present, the specific surface area of ​​graphene materials (200-1200 m2 /g) is far from the theoretical prediction. How to control the texture of graphene so that the surface of graphene can be completely infiltrated by electrolyte solution is the current important Question. The characteristics of graphene are similar to those of activated carbon and graphite materials. If used as an electrode material, it can be compatible with existing supercapacitors and lithium ion batteries. Graphene materials have electrical and thermal conductivity properties, and can form a thickness-regulated graphene film, which can construct very good thin-film batteries and energy storage devices. As a basic material of sp2 hybrid materials, graphene can be constructed by secondary modification and recombination, and by constructing “nano-architectureâ€. By optimizing the structure, materials with high storage capacity can be obtained. We have cooperated with the Jinggulong Group of Tohoku University in Japan to study that single-walled porous carbon obtained by twisting a single layer of graphene sheets can be prepared in the microporous pores of molecular sieves, and very good high-power characteristics can be obtained by heat treatment. In summary, graphene materials have excellent energy storage properties and also show good application prospects. At present, the research of graphene has yet to be deepened. After systematic research and development to solve scientific problems and technological problems, it is expected to become an electrode material with huge market potential.
Hdpe rod and sheet is a high crystallinity and non-polarity thermoplastic resin,HDPE Rod and sheet has good chemical stability, which can resist corrosion of most acid,alkali,organic solution and hot water; good electric insulation property and is easy to weld.
AHD is the first to produce ultra-thick PE plate (with a thickness up to 200 mm) in China,whom started to research,develop and produce HDPE rod and sheet in 2002.
HDPE rod and sheet are widely applied to drinking water/sewerage pipe,hot water pipe,conveying container,pump and valve part, medical appliance,seal part,cutting plate and sliding material in chemical industry,machinery,power plant,garment,packaging and food industries.
HDPE Sheet, PE sheet,Polyethylene Sheet,pe plastic sheet pe rod,HDPE Rod Shenzhen Anheda Plastic Products Co.,Ltd , https://www.ahdplastic.com